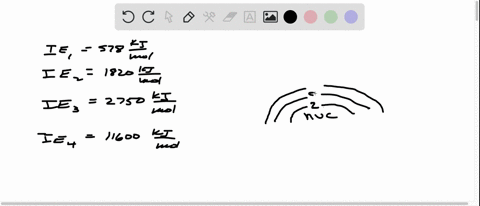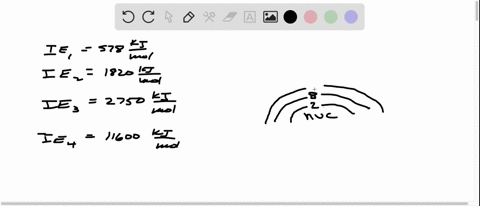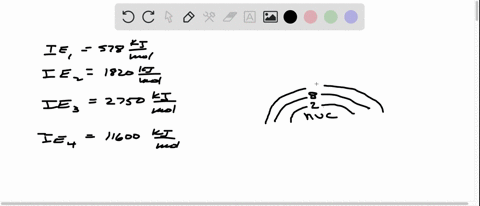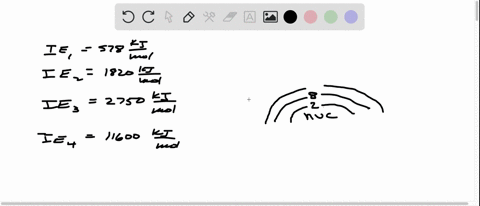
Consider the following set of successive ionization energies: IE1=578 kJ/molIE2=1,820 kJ/molIE3=2,750 kJ/molIE4=11,600 kJ/mol This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: To which third-period element do these ionization values belong?
Solved To which third-period element do these ionization | Chegg.com
Jun 12, 2023These ionization values are listed in the table given below:Element: Mg (Magnesium)First ionization energy: 738 kJ/molSecond ionization energy: 1450 kJ/molThird ionization energy: 7732.7 kJ/mol For a neutral atom, the first ionization energy (IE1) is the amount of energy required to remove an electron from the outermost shell.
 Download Image
Download Image07/10/2023 Chemistry High School answer answered Consider this set of successive ionization energies: ie1 = 578 kj>mol ie2 = 1820 kj>mol ie3 = 2750 kj>mol ie4 = 11,600 kj>mol to which third-period element do these ionization values belong? a. P b. Mg c. AI d. Si Advertisement AI-generated answer
Source Image: www.chegg.com
Download Image
Notes on Periodic Classification of Elements (BSc and Integrated Standard) To which third-period element do these ionization values belong Spell out the full name of the element. Submit Request Answer. BUY. Chemistry for Engineering Students. 4th Edition. ISBN: 9781337398909. Author: Lawrence S. Brown, Tom Holme. Publisher: Cengage Learning. expand_more.

Source Image: www.yumpu.com
Download Image
To Which Third-Period Element Do These Ionization Values Belong
To which third-period element do these ionization values belong Spell out the full name of the element. Submit Request Answer. BUY. Chemistry for Engineering Students. 4th Edition. ISBN: 9781337398909. Author: Lawrence S. Brown, Tom Holme. Publisher: Cengage Learning. expand_more. Jun 16, 2023Consider this set of successive ionization energies: IE1 = 578 kJ>mol IE2 = 1820 kJ>mol IE3 = 2750 kJ>mol IE4 = 11,600 kJ>mol To which third-period element do these ionization values belong? Consider this set of successive ionization energies: IE1 = 578 kJ>mol IE2 = 1820 kJ>mol IE3 = 2750 kJ>mol IE4 = 11,600 kJ>mol
WORKSHEET, PERIODIC TABLE (CHAPTER 6) – Avon Chemistry
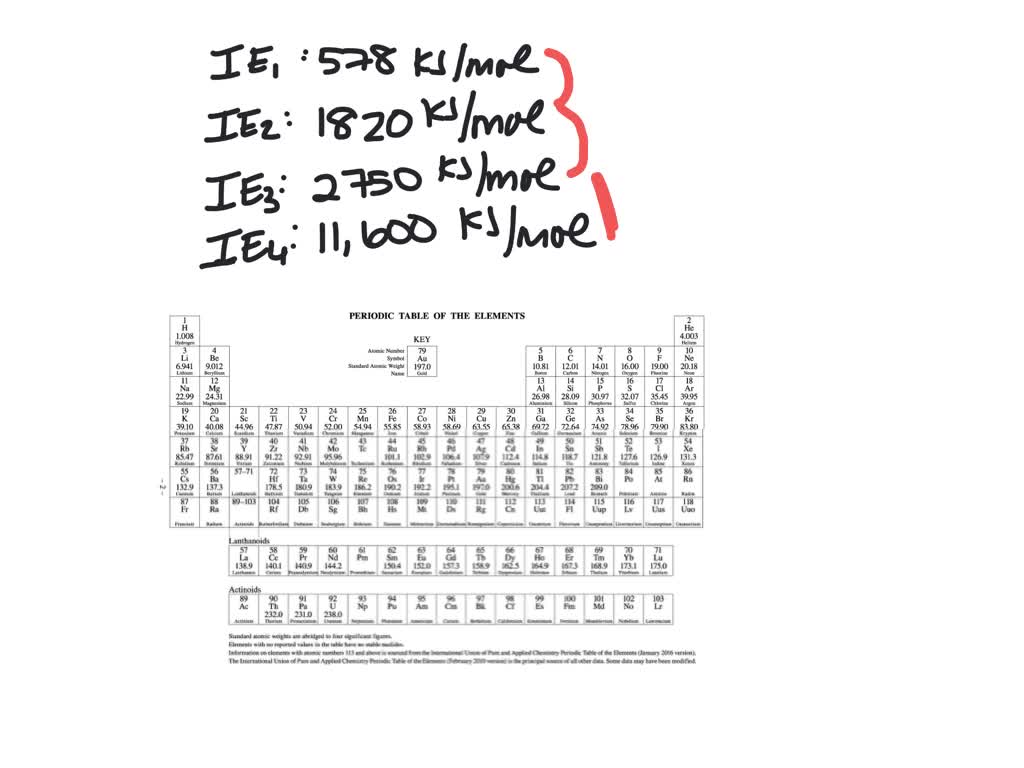
Consider this set of ionization energies. IE1 = 578 kJ>mol IE2 = 1820 kJ>mol IE3 = 2750 kJ>mol IE4 = 11,600 kJ>mol To which third-period element do these ionization values belong? The first ionization enthalpy (∆i H ) valuesof the third period elements, Na, Mg and Si

Source Image: m.youtube.com
Download Image
⏩SOLVED:Consider this set of successive ionization energies: … | Numerade Consider this set of ionization energies. IE1 = 578 kJ>mol IE2 = 1820 kJ>mol IE3 = 2750 kJ>mol IE4 = 11,600 kJ>mol To which third-period element do these ionization values belong?
Source Image: www.numerade.com
Download Image
Solved To which third-period element do these ionization | Chegg.com Consider the following set of successive ionization energies: IE1=578 kJ/molIE2=1,820 kJ/molIE3=2,750 kJ/molIE4=11,600 kJ/mol This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: To which third-period element do these ionization values belong?

Source Image: www.chegg.com
Download Image
Notes on Periodic Classification of Elements (BSc and Integrated Standard) 07/10/2023 Chemistry High School answer answered Consider this set of successive ionization energies: ie1 = 578 kj>mol ie2 = 1820 kj>mol ie3 = 2750 kj>mol ie4 = 11,600 kj>mol to which third-period element do these ionization values belong? a. P b. Mg c. AI d. Si Advertisement AI-generated answer

Source Image: www.pratapsir.com
Download Image
Problem 3.0 The first ionization enthalpy (4,H) values of the third period elements, Na, Mg and Si are respectively 496, 737 and 786 kJ mor”. Predict whether the first A H value Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider this set of ionization energies. $IE_1 = 578 kJ / mol$, $IE_2 = 1820 kJ / mol$, $IE_3 = 2750 kJ / mol$, $IE_4 = 11600 kJ / mol$ To which third-period element do these ionization values belong?.

Source Image: www.toppr.com
Download Image
SOLUTION: Btc213 lecture 6 – Studypool To which third-period element do these ionization values belong Spell out the full name of the element. Submit Request Answer. BUY. Chemistry for Engineering Students. 4th Edition. ISBN: 9781337398909. Author: Lawrence S. Brown, Tom Holme. Publisher: Cengage Learning. expand_more.

Source Image: www.studypool.com
Download Image
Group 3A Elements | Facts, Properties & Metals – Video & Lesson Transcript | Study.com Jun 16, 2023Consider this set of successive ionization energies: IE1 = 578 kJ>mol IE2 = 1820 kJ>mol IE3 = 2750 kJ>mol IE4 = 11,600 kJ>mol To which third-period element do these ionization values belong? Consider this set of successive ionization energies: IE1 = 578 kJ>mol IE2 = 1820 kJ>mol IE3 = 2750 kJ>mol IE4 = 11,600 kJ>mol

Source Image: study.com
Download Image
⏩SOLVED:Consider this set of successive ionization energies: … | Numerade
Group 3A Elements | Facts, Properties & Metals – Video & Lesson Transcript | Study.com Jun 12, 2023These ionization values are listed in the table given below:Element: Mg (Magnesium)First ionization energy: 738 kJ/molSecond ionization energy: 1450 kJ/molThird ionization energy: 7732.7 kJ/mol For a neutral atom, the first ionization energy (IE1) is the amount of energy required to remove an electron from the outermost shell.
Notes on Periodic Classification of Elements (BSc and Integrated Standard) SOLUTION: Btc213 lecture 6 – Studypool Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider this set of ionization energies. $IE_1 = 578 kJ / mol$, $IE_2 = 1820 kJ / mol$, $IE_3 = 2750 kJ / mol$, $IE_4 = 11600 kJ / mol$ To which third-period element do these ionization values belong?.